01052021 The SARS-CoV-2 Protein Antigen assay is intended for Research Use Only to detect the presence or absence of the nucleocapsid protein antigen from the SARS-CoV-2 virus in nasopharyngeal swab specimens. The two main antigens for coronavirus SARS-CoV-2 are the spike S protein and the nucleocapsid N protein.
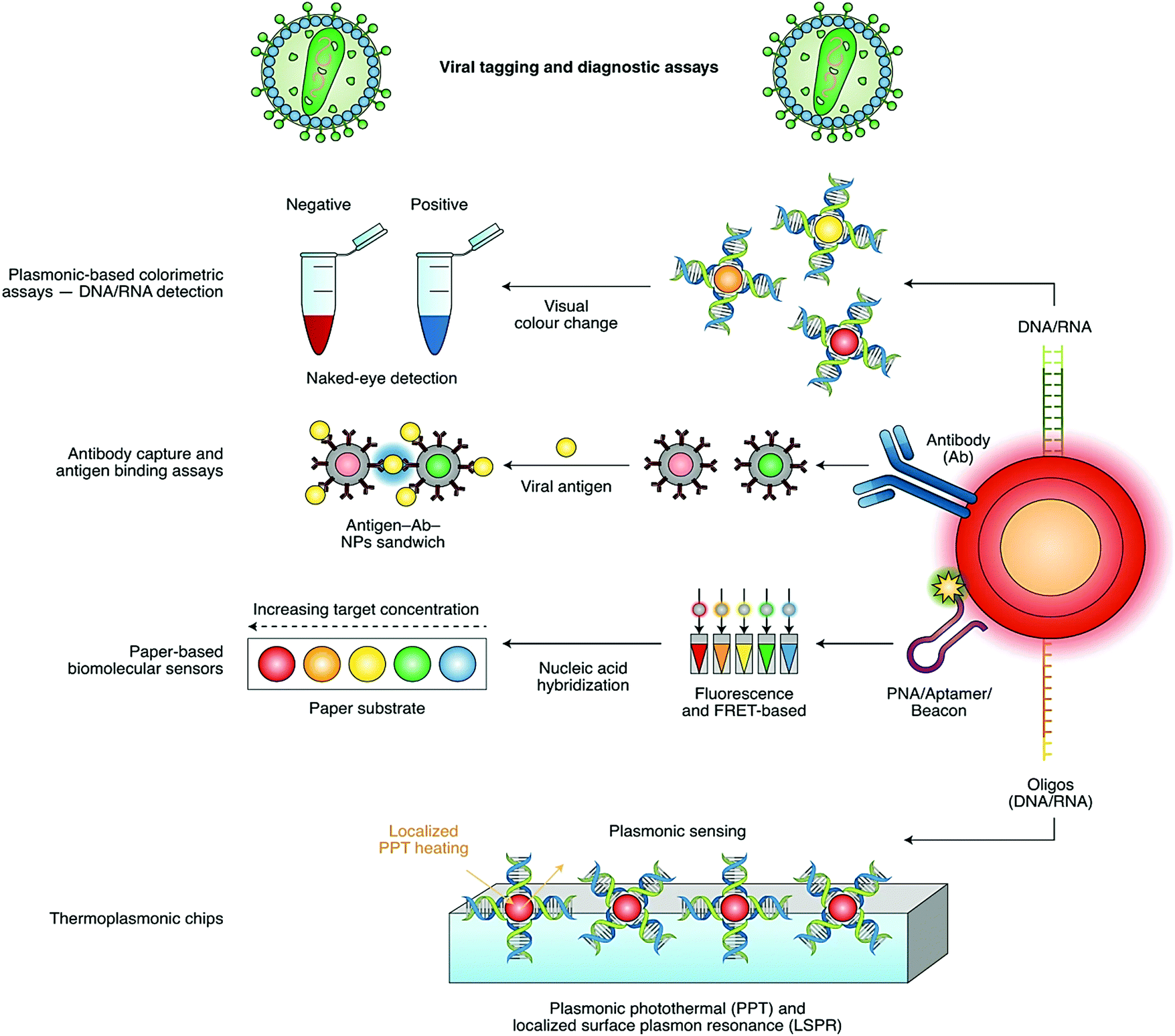
Nano And Biosensors For The Detection Of Sars Cov 2 Challenges And Opportunities Materials Advances Rsc Publishing
They are performed on blood samples collected by venipuncture.

N protein antigen test. 21072021 The antigen test they administer is described as. Colloidal gold immunochromatography assay was used to detect serum N protein antibodies in the above populations. 18062020 Nucleocapsid protein is a crucial part of SARS-CoV-2.
31082020 The N protein plays an important role in both SARS-CoV and SARS-CoV-2 infection by packaging viral RNA and aiding in the release of additional viral particles from infected cells. SARS-CoV-2 nucleocapsid protein NP is an ideal target for viral antigen-based detection. N-protein has also been tested in its use as a protective vaccine against SARS-CoV infection.
MethodsSerum N protein level in SARS-COV-2 infected patients and non-SARS-COV-2 infected population was measured by enzyme-linked immunosorbent assay ELISA double antibody sandwich assay. Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens I called ATN and they didnt know what the N stood for either. This nucleocapsid protein or N protein is elevated in respiratory fluids during the initial acute phase of the infection.
With C t value 40 as the cutoff of NA testing the sensitivity specificity and percentage agreement of the FIC assay was 756. 02062021 The Simoa SARS-CoV-2 N Protein Antigen Test is an automated paramagnetic microbead-based immunoassay intended for the qualitative detection of the nucleocapsid protein N protein antigen from SARS-CoV-2 in nasopharyngeal swab specimens collected in Huachenyang iClean Viral Transport Medium VTM CDCs formulation of VTM normal saline or phosphate. 26032021 The Simoa N-protein antigen test represents a robust SARS-CoV-2 detection tool in multiple types of sample matrix.
EUROIMMUN was among the first companies to release an antibody assay based on the S1 subunit of the spike protein which could be fully automated using their technologies. Results Receiver operating. SARS-CoV-2 N Protein Antigen Test.
IgM antibody tests Against Spike Protein or Nucleocapsid N Protein and IgG antibody test may either be ordered together or separately. Intended use The kit is used for the qualitative detection of new coronavirus nucleocapsid N antigen in human throat swab samples in vitro. The presence of the antigen will directly indicate that the tested individual carries the virus.
A rapid and convenient method was developed based on fluorescence immunochromatographic FIC assay to detect the SARS-CoV-2 NP antigen. The Simoa SARS-CoV-2 N Protein Antigen Test is an automated paramagnetic microbead-based immunoassay intended for the qualitative detection of the nucleocapsid protein N protein antigen from SARS-CoV-2 in nasopharyngeal swab specimens collected in iClean HuachenyangViral Transport Medium VTM CDCs formulation of VTM normal saline or phosphate buffered saline. Of 251 participants 992 included in the diagnostic accuracy analysis 201 801 had a C t value of 40.
These tests are designed to detect antibodies IgM and IgG that our immune system produces against the SARS-CoV-2 virus that causes COVID-19 disease. A total of 253 participants were enrolled. Two participants were excluded from the analyses because of invalid NP testing results.
10092020 Many companies have conducted millions of tests to detect antibodies against the N protein potentially misleading the antibody positive individuals regarding their plausible long-term immunity. The antigen test is an alternative diagnostic method that relies on the immunodetection of the viral antigens in biological samples. So it could be a potential vaccine candidate in the effort to.
This test is to be performed only using nasopharyngeal swab specimens collected from individuals in transport media who are suspected. While most tests detect antibodies against either S or N proteins some tests can detect antibodies against both immunodominant proteins multiplex assays. N-protein has been one of the top target for development of IgM ELISA for diagnostic purpose.
The nucleocapsid protein is always selected to capture IgGIgM in the COVID-19 serological kits because of its high immunogenicity. 12012021 Designed to run on the Simoa HD-X analyser the antigen test will help identify the presence of SARS-CoV-2 virus nucleocapsid protein or N protein which is said to be inflated in respiratory fluids during the initial acute phase of the infection. 17032021 Based on the reagents total antibody Ig can be detected or IgG and IgM can be detected separately.
IVD test for the detection of SARS-CoV-2 Nucleocapsid Protein Antigen presence in a human oropharyngeal swab sample ie the demonstration possible COVID19 infection. However the accuracy of.

Evaluation Of A Novel Antigen Based Rapid Detection Test For The Diagnosis Of Sars Cov 2 In Respiratory Samples International Journal Of Infectious Diseases
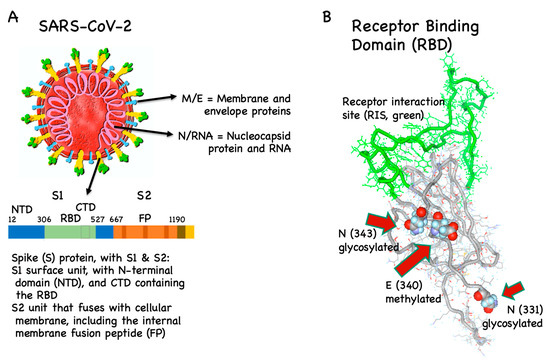
Vaccines Free Full Text Covid 19 Mechanisms Of Vaccination And Immunity Html
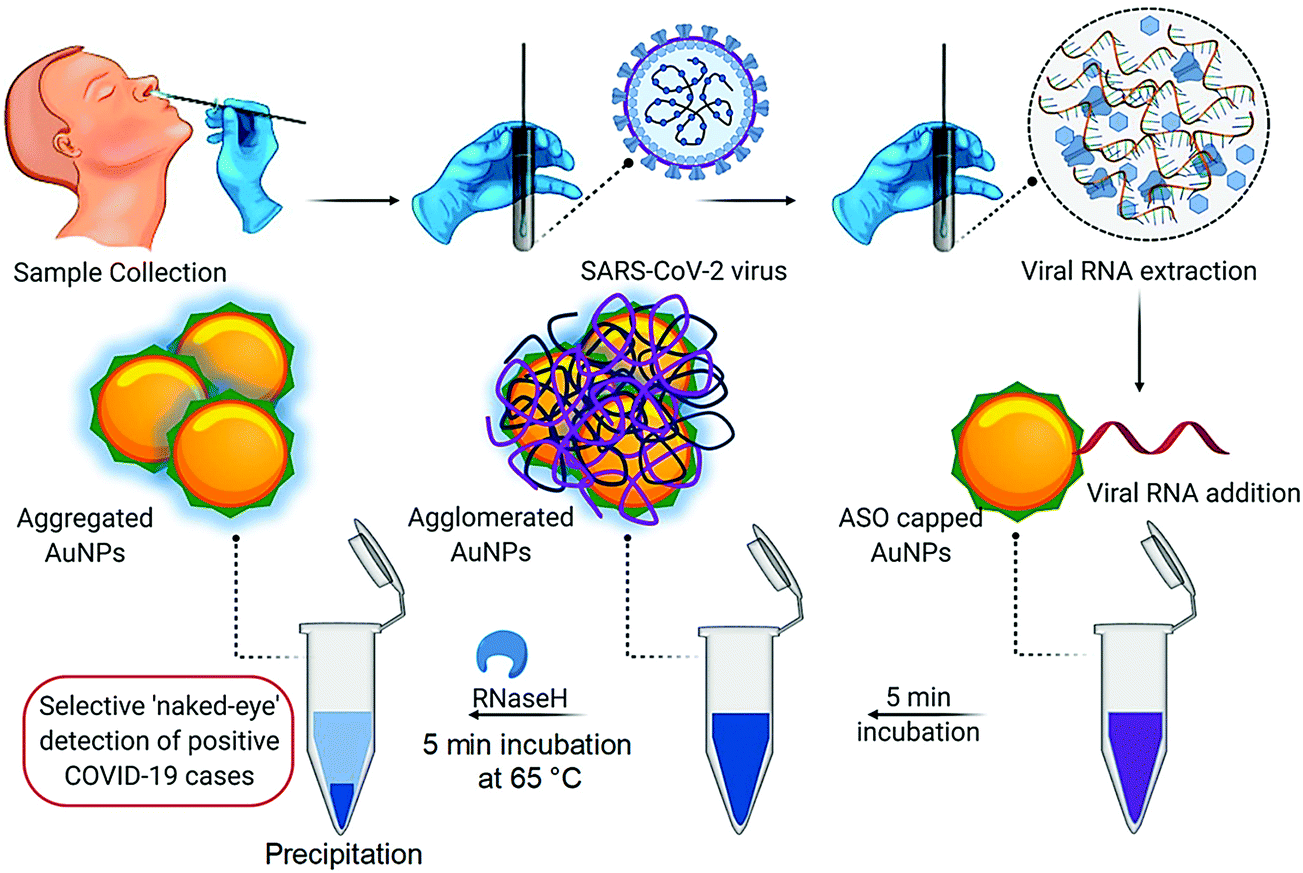
Nano And Biosensors For The Detection Of Sars Cov 2 Challenges And Opportunities Materials Advances Rsc Publishing Doi 10 1039 D0ma00702a

Coronavirus Disease Covid 19 Antibody Test For Providers Labcorp

Implementation Of Antigen Rdt Ag Rdt To Detect Covid 19 Cases In Indonesia

Evaluation Of A Novel Antigen Based Rapid Detection Test For The Diagnosis Of Sars Cov 2 In Respiratory Samples International Journal Of Infectious Diseases

Swab Antigen Dan Rapid Test Antigen Beda Atau Sama
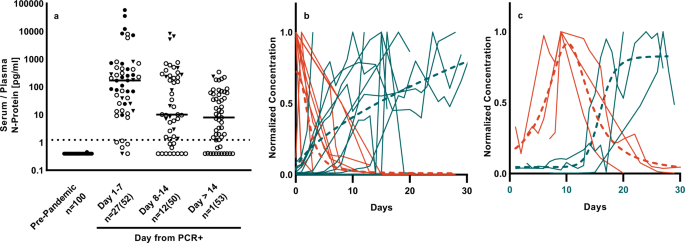
N Protein Presents Early In Blood Dried Blood And Saliva During Asymptomatic And Symptomatic Sars Cov 2 Infection Nature Communications

Adjuvanted Sars Cov 2 Spike Protein Elicits Neutralizing Antibodies And Cd4 T Cell Responses After A Single Immunization In Mice Ebiomedicine

0 comments:
Post a Comment