Ad Bestll kosttillskott fr lgre priser hos MM Sports. This is the protocol required by the Chinese Consulate.
Sars Cov 2 Igg Antibody Test Receives Fda Emergency Use Authorization Healthcare In Europe Com
A series of blood samples were collected along the disease course from the same patient including 11 ICU patients and 27 non-ICU patients for longitudinal analysis.

N protein igm test. COVID-19 PCR NAAT RT-PCR Antibody Test IgM S Protein. They are often measured to help diagnose different conditions such as infections immunodeficiency autoimmune disease and certain types of cancer. Many companies have conducted millions of tests to detect antibodies against the N protein potentially misleading the antibody positive individuals regarding their plausible long-term.
30102020 IgM COVID-19 serum venous blood antibody test. Levels Immunoglobulin M antibodies IgM are our first-line defense against a broad range of infections. Choose between 14-hour or 24-hour turnaround time.
The FDA issued an emergency use authorization EUA for the test kits utilized in this test. Food and Drug Administration authorized serology test to detect SARS-CoV-2 IgM antibody 1 utilizing S protein spike protein as the antigen target. Test result report provided to the Testee and submitted to the Chinese Consulate General required Test result report that complies with the requirements set forth by the Chinese Consulate.
Snabb leverans och kvalitetsgaranti. 18062020 The nucleocapsid protein is always selected to capture IgGIgM in the COVID-19 serological kits because of its high immunogenicity. Further analysis showed that in comparison to race CCI lymphocyte counts and.
Ver 100 olika varumrken inom kosttillskott trningsklder. So it could be a potential vaccine candidate in the effort to. N-protein has been one of the top target for development of IgM ELISA for diagnostic purpose.
07042021 Fishers exact test showed that the proportions of either IgM or IgG antibodies in patients that detected S2 and N were signficantly greater than S1 at p 0001 and p 0013 respectively and the proportions of either IgM or IgG antibodies detecting S1 S2 and N were significantly more than RBD at p. 01122019 IgM Immunoglobulin M Antibodies. These two tests utilizes US.
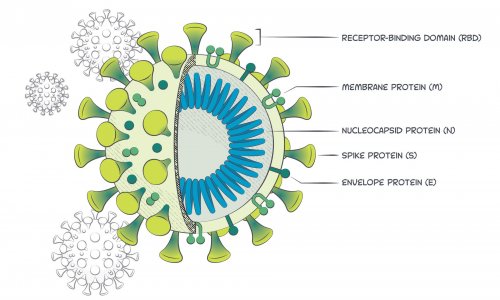
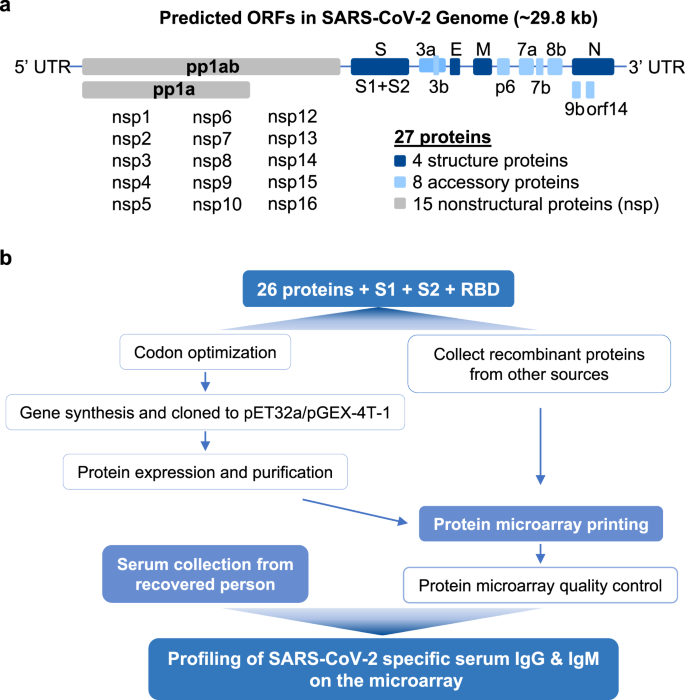
Coated with reagents for the detection of IgM anti-SARS CoV 2 N nucleocapsid and S spike protein and the C line is pre-coated with quality control. As the vital component in the serological test. The levels of IgM and IgG specific to N and S protein were detected by ELISA.
These tests are designed to detect antibodies IgM and IgG that our immune system produces against the SARS-CoV-2 virus that causes COVID-19 disease. Snabb leverans och kvalitetsgaranti. N Protein Expedited test services.
Ver 100 olika varumrken inom kosttillskott trningsklder. And 2 utilizing N protein nucleocapsid protein as the antigen target. They are performed on blood samples collected by venipuncture.
N and S specific IgM and IgG N-IgM N-IgG S-IgM S-IgG in non-ICU patients increased after symptom. IgM antibody tests Against Spike Protein or Nucleocapsid N Protein and IgG antibody test may either be ordered together or separately. For vaccinated travelers who test positive for IgM antibodies we will run the N-Protein IgM test 75 extra.
28092020 Anti-N protein IgG was positive in 55 55 patients at the time of admission. This test utilizes US. 10092020 It is important to be cautious when using serology tests that are based on the N protein for addressing questions that are related to determining potential COVID-19 immunity.
The FDA issued an emergency use authorization EUA for the SARS-CoV-2 IgGIgM serology test kit utilized in this test. Food and Drug Administration authorized serology tests to detect SARS-CoV-2 IgM antibody utilizing S protein spike protein and N protein nucleocapsid protein as the antigen targets. Expedited service with results delivered by 1700 PM PST the next day after collection.
If needed passengers can choose a laboratory on the list that provides IgM antibody test against N protein and the test must also be sampled at the departure place within 48 hours before boarding. N-protein has also been tested in its use as a protective vaccine against SARS-CoV infection. Ad Bestll kosttillskott fr lgre priser hos MM Sports.
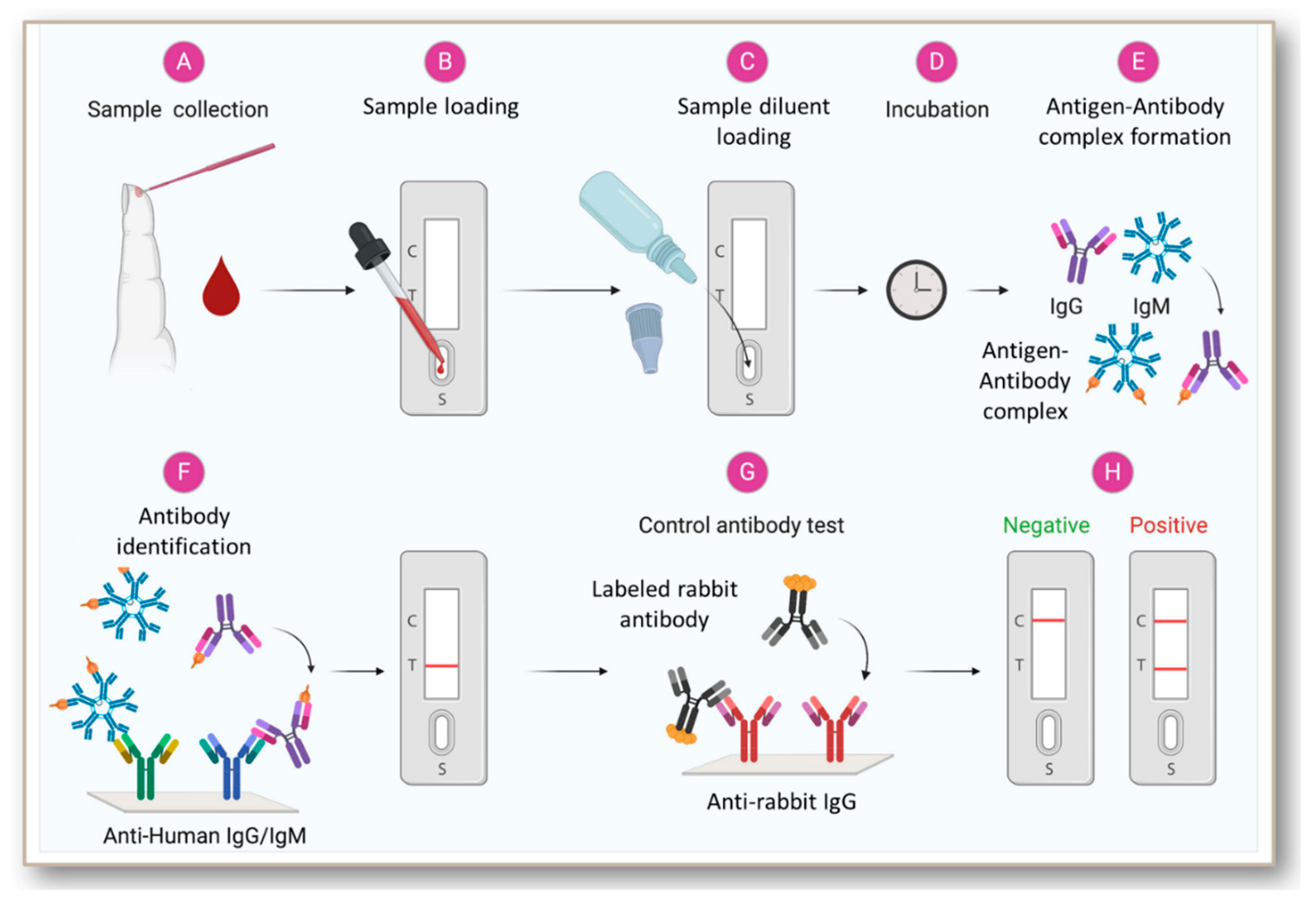
A plastic shell and a reagent strip which is composed of a sample pad a colloidal gold pad coated with recombinant 2019-nCoV N protein and S protein nitrocellulose membrane with two test lines these two lines are coated with anti-human IgM and IgG antibody respectively the control line coated with anti recombinant protein tag protein.

Diagnostic Value And Dynamic Variance Of Serum Antibody In Coronavirus Disease 2019 International Journal Of Infectious Diseases

Coronavirus Covid 19 Igg Igm Sars Cov 2 2019 Ncov Kits Products Mybiosource

False Positive Results Of Sars Cov 2 Igm Igg Antibody Tests In Sera Stored Before The 2020 Pandemic In Italy International Journal Of Infectious Diseases

Antibody Testing Against Covid 19 Igm S And N And Igg
Jcm Free Full Text Rapid Antibody Based Covid 19 Mass Surveillance Relevance Challenges And Prospects In A Pandemic And Post Pandemic World Html

Products For Covid 19 Research Diagnostics Lucerna Chem Ag

Dynamics Of Anti Sars Cov 2 Igm And Igg Antibodies Among Covid 19 Patients Journal Of Infection
Sars Cov 2 Proteome Microarray For Global Profiling Of Covid 19 Specific Igg And Igm Responses Nature Communications

Rapid Test Covid 19 Igg Igm Cellex Qsars Cov 2 Vaxcorp Indonesia

0 comments:
Post a Comment